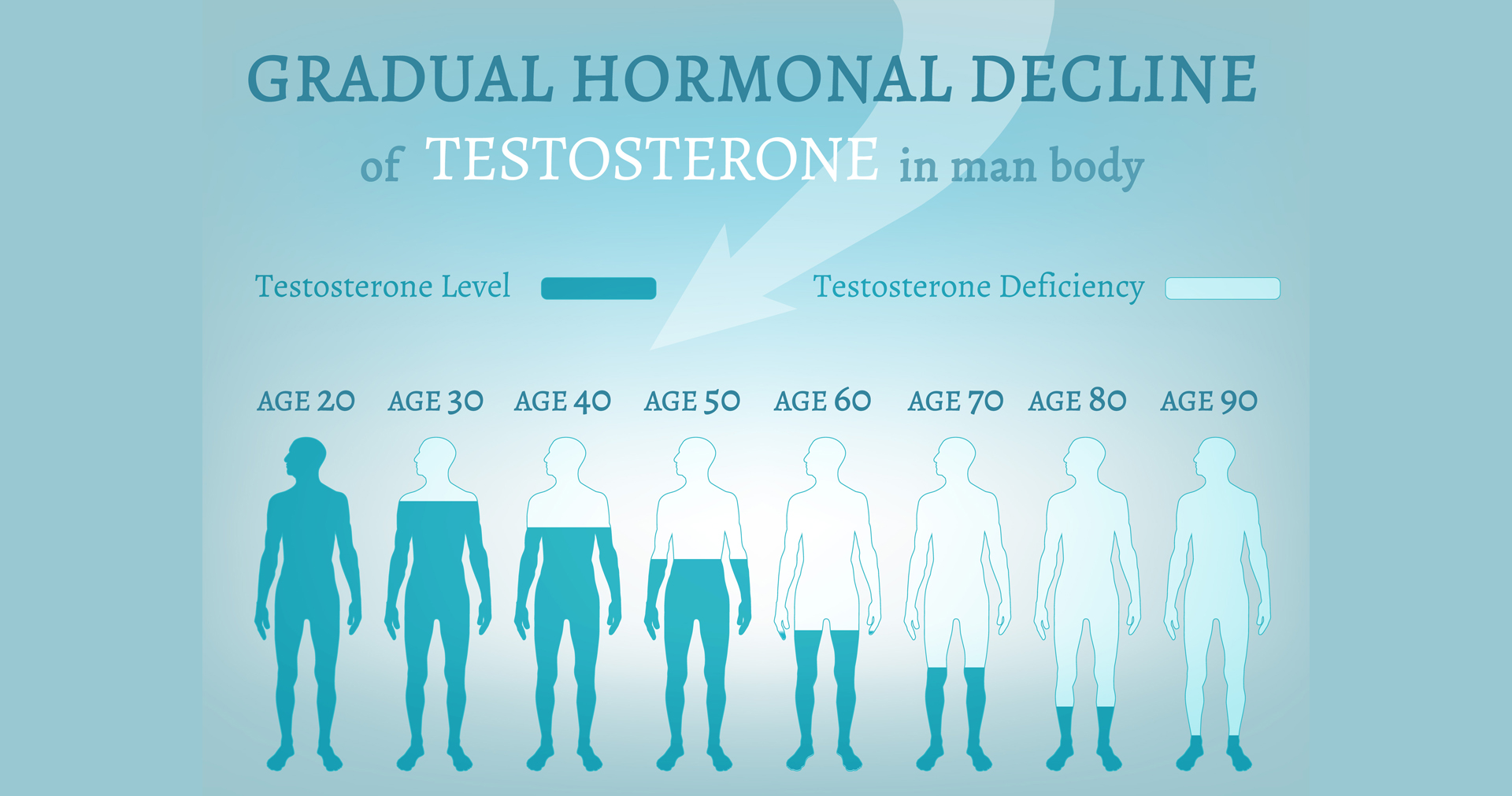
Bioelectric treatment of Low Testosterone
TestiStim is an early stage startup focused on bioelectric and biologics treatment of infertility and low testosterone levels in man.
TestiStim
Early Stage Startup
TestiStim is an early stage startup focused on bioelectric and biologics treatment of infertility and low testosterone levels in man.
The device will used to regenerate testicles to restore their full performance in creating sperm and managing via neuro modulation testosterone levels.

Bioelectric Stimulations
The technology is envisioned to sense and monitor testosterone levels and adjust them to a healthy level via programmed bioelectric stimulations not only directly into the testes but also via the nervous system. The bioelectric stimulation can be delivered via invasive pacing lead catheters, wireless programmed micro implants or a variety of non-invasive methods including temporal interference or simple conductive under garments.

Micro Infusion Pump
For severe recovery cases a re-fillable micro infusion pump will be used that is re-filled daily, weekly or monthly as required with a mixed composition of modified or selected stem cells and a cocktail of support factors such as exosomes, micro RNA gel, selected growth factors, selected alkaloids such as tetraharmine, PRF, amniotic fluid, nutrient hydrogel and organ specific matrix.


